It’s 7:00 am, and you’re pushing your way to the front of the crowd, looking up you see hundreds of hands moving furiously, vying for a chance to buy one of the many lucrative stocks. This is the New York Stock Exchange, or the dramatized version you see in 90s movies. Each set of hands has different strategies and is fighting for other commodities; some want to buy 10000 barrels of crude oil, others are interested in the newest tech giant’s stock, however, all of them want to earn as much money as possible! This scene isn’t just happening in high-rise buildings all around our world, but also in the complex architecture of our gut.
Sounds unbelievable? Well, it's 7:00 am and you just ate breakfast, marking the opening of your gut’s stock market. Each second there are trillions of little traders in your gut, doing all kinds of activities. They have some enemies which they compete with, but there are also some friends who cooperate together, however all are egoistic in the end. Even though they closely resemble the stock traders, their goal is different - instead of fighting for money, the little guys are fighting to replicate as much as possible.
All this hard work in your guts is very worth it - if the process is balanced, it ensures your health and well-being. On the other hand, if their way of life is disrupted, it’s as if the economy crashed. Unhappy little traders can be linked, as researchers have found in various reviews, to many of the modern-day killers: think obesity, diabetes, and even Parkinson’s disease.

These little traders are actually microorganisms and come in all different shapes and sizes, like bacteria, fungi, and even viruses. The community of them living in our gut we call the gut microbiome. But nearly every part of life has its own microbiome (collection of different traders): your mouth, hands, fridge, and even your laptop screen! So everywhere you look there’s probably a completely unique community of microorganisms interacting with each other and their surrounding environment.
Now back to our guts, these microorganisms help us digest our food, breaking it down into simple building blocks our bodies can use for energy and other functions. Additionally, these microorganisms help facilitate our immune system, keeping us healthy and stopping unwanted invaders in their tracks. Even our mood is partially determined in our gut, as the majority of the feel-good chemicals like serotonin and dopamine are produced there. Researchers have found for example that the gut provides approximately 95% of total body serotonin, most of which exists in plasma. From this, it should be clear that we want a happy and healthy gut. But what does that actually mean?
In the simplest terms, it means a gut with a diverse range of microorganisms working and competing in a balanced way. But what these interactions look like in the gut is much more complex. So, we need a clever representation to reflect this. This article will show how networks can act as a representation of the microbiome as a population of microorganisms and their interactions.
A map of the microbiome
Researchers studying the microbiome of individuals have already established links between the microorganisms present in samples and specific diseases or illnesses, such as obesity, IBS (irritable bowel syndrome), COVID-19, and even depression.
To figure out what the microorganisms are doing in our gut is very challenging for one key reason - we can’t see the little guys! So we must go about this less directly by looking at what leaves our system. This sample acts as a reflection of the gut microbiome and will be analyzed, via DNA sequencing methods, to find out what microorganisms are present.
The relative amounts of each microorganism can also be determined, which can provide additional insights. We may find that individuals with a certain disease or illness have microbiomes that differ from healthy individuals, their microbiome may be lacking certain microorganisms or may have certain microorganisms that aren’t present in healthy individuals. From large amounts of these studies, we can begin to infer what a healthy microbiome looks like.
Personal Investigators and Interaction Networks
This first analysis has only told us what types of little traders are present in our microbiome, but these little guys are not sitting in isolated cubicles, interacting with no one. They are living rich lives full of interactions, fighting with one another for resources, and being part of long chains of production: using someone’s trash to fuel their lives and having their trash fuel another. If we want to get more of the full story, we need to look at the interactions between these microorganisms. However getting this information is difficult - monitoring change requires huge resources and constant measurements. This is where networks come in! Namely, we can construct a network to help us understand the complex interactions between different microorganisms.
Microorganisms in our microbiome are living rich lives full of interactions! We can construct a network to understand them.
To begin constructing such a network, we must infer interactions from the data we’ve already collected. Think of yourself as a private investigator tasked to find out what stock market traders are conspiring together, but the only information you have is pictures of the stock exchange floor on different days. The stock exchange is a bustling place, and each day brings new people making it very difficult to tell who could be conspiring. But you begin to notice a pattern, on certain days there are a few traders who are usually standing near each other. Could they be conspiring or is this due to chance? This is hard to say, but still, you note down the suspicious names, drawing lines between the individuals that always seem to appear next to each other.
Unknowingly, you’ve just created a network. A network is a collection of items, called nodes connected with edges. In this case, the nodes are the stock traders, connected with edges if they are often seen near eacht other. Networks can be weighted if edges have values, or weights, associated with the strength of the interaction between two nodes. They can also be directed if the connection between two nodes only runs in one direction.
Microbiome researchers follow a similar line of reasoning as the private investigator. They look at the microorganisms they’ve identified and their relative amounts and identify microorganisms that seem to occur together more often than is likely due to chance. They compile this information into a network where the nodes are microorganisms with edges between them if they often occur together. These edges are weighted to take into account the relative frequency of the two organisms appearing together in a sample. This is called a co-occurrence network.
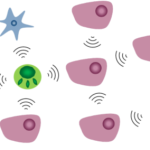
It’s important to note this network gives you no information about actual interactions between microorganisms, but only whether they often occur together. Hence an important question that arises is: how do microorganisms interact with one another? Well, they can cooperate, either by altruistically helping another species or by opportunistically happening to help. Interestingly, for evolutionary stability, there need to be benefits for both sides coming from the cooperation - without it, the collaboration will quickly be discontinued. Microorganisms are very similar to us humans in that regard - no one is entirely selfless. Naturally, there is also a lot of competition. And then there are the truly hated relations, which are entirely one-sided and prey on weaker organisms, like exploitation, predation, and parasitism. In the microbiome, these usually happen when a specific species gets a severe advantage over others in a short time. To get closer to understanding and characterizing the interactions happening right now in our gut we must create a different network, the so-called interaction network.
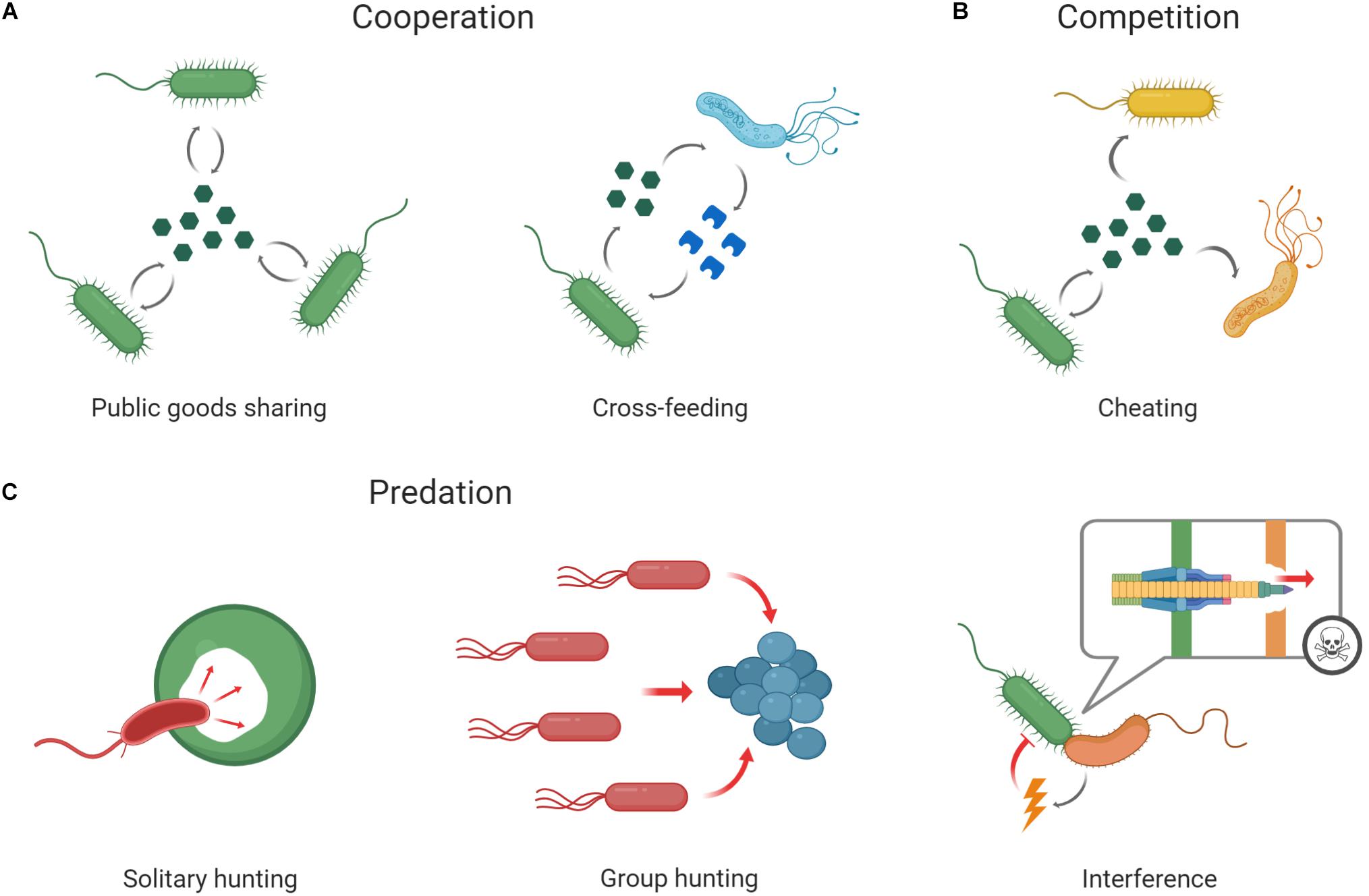
Figure: Social interactions among microbes. Microbes frequently interact with clonemates or other microbes of the same or different species (represented by microbes of different colors and shapes, respectively). This picture and description were taken from the original article by Alexandre R. T. Figueiredo and Jos Kramer published in Frontiers in Ecology and Evolution.
To be able to characterize interactions in a general sense we can use information of how the composition of the microbiome changes with time. By collecting samples over days and months we can get this information. Afterwards, we use statistical tools to figure out how the relative proportion of one microorganism changes in response to another. From this, we can infer interactions; cooperation or mutualistic interactions occur if two microorganisms increase in response to one another. Similarly, competitive interactions occur if two microorganisms decrease in response to one another. We can compile this information into another network, and call this an interaction network, signifying the competitive interactions with negative weights and cooperative interactions with positive weights. These networks can be directed if the interaction appears to only be in one direction, for example in a parasite relationship.
Why compete?
Until now we’ve characterized the different types of interactions in our gut, now what? Well, this information about the different types of interactions can tell us a lot about the health of our guts. Similar to the benefits of the daily fights between traders in the stock exchange, organisms in the microbiome should also compete. We want our guts to be filled with a diverse range of mutually beneficial and competitive interactions, a perfect blend of friends, frenemies, and enemies to keep our guts active and our body on its toes. This might be surprising, but in order to stay healthy you want to have a cut-throat atmosphere microbiome in your gut! Why? Well, there are multiple benefits and network science helps us understand them.
We want our guts to be filled with a diverse range of mutually beneficial and competitive interactions, a perfect blend of friends, frenemies, and enemies to keep our guts active and body on its toes
Firstly, a study published in Science led to an unexpected conclusion - competition goes hand in hand with network stability - so no more major compositional changes happening in your stomach! Stability is essential in your gut to ensure smooth operations and health. After all, imagine a game of chess between the two best grandmasters in the world. Due to their incredible and unparalleled skill, it is difficult for both to make moves - the game is usually slow and calculated. You can imagine a sort of stability both on the chessboard in a specific game (of course there are minor victories, but usually we cannot speak of a decisive defeat), but also in the leaderboards - most of the best players do not move in rankings that much.
A similar thing happens in your gut with enough competition - obviously, there are some small changes because the system is not static, however, the specific groups of organisms are held at an impasse most of the time.
Let's discuss in more depth the findings in this article about all the costs and benefits of microbial competition. In our gut, microbes fighting for resources are less likely to cooperate. Since microbes are fighting with each other the overall benefits for you are smaller than if they worked together. From a bird's eye view, multiple species fighting for the same resources means that these resources will always be used, and the jobs that are needed will always get done, even as populations change. This is what the paper found, they analyzed the communities (smaller parts) in the network on a local and global level to conclude that too much cooperation destabilizes the microbiome networks and results in difficulty in returning to an equilibrium. Additionally the more diversity in species, the harder it is to achieve stability, as each microorganism has its own unique needs. But this paper found that increasing diversity need not be negative, as long as there is also an increase in competition - stability can then still be achieved.
Changes in the network can shed light
After discussing that cooperation and competition are both important for stability we will turn to another important question. Namely, what can we learn about the microbiome when observing changes in the interaction networks? What do changes in the microbiome actually mean for the bacteria in your body? Luckily, another article helps us answer these questions. Most strategies that microbes employ are evident in the changes of the network over time, this is the interaction network we talked about earlier - just like in the NYC stock exchange, the exact nature of interactions between actors influences the playing field. So what do these phenomena usually mean? There are three important cases:
- Large changes in node presence and connectivity: this represents the most basic form of competition (and probably also most common), focusing on fighting for the same resources or trying to eliminate opponents through e.g. toxic antibiotics. Through this mechanism, some microbial populations decrease, whereas others flourish, in turn leading to changes in how important the nodes representing them in the network become.
- Community development in the network: usually caused by indirect effects, groups or communities of microbes working together. Think of this as legal regulators in the stock exchange, influencing how traders work or groups of traders artificially increasing prices to control the market. After all, working in a group produces more impact than working alone. However, these communities can also help elevate the positions of its members in the context of the entire network.
- Cycles: mean that a group of microbes is in a cycle of dominance, without a clear winner - the competition will go on to infinity and beyond because it is almost impossible for a member to break out. Thus, there is no clear winner, instead everyone takes turns being on top.
These are only some examples of competition mechanisms between microbes, but they clearly showcase the strength of using networks in analyzing our guts! Who knows, maybe in the future, they can also help biologists solve many of our health problems.
Having explored some exciting ideas, it is important to reflect on the relevance of the emerging field. The microbiome is not just a craze that a handful of scientists are interested in. It has great implications not only for our health but also understanding of biological systems. After all, 70% of our immune system inhabits our guts. At the same time, microbial communities are complex and largely unexplored.
We’ve seen how to construct two types of networks; co-occurrence networks, from which correlations may be determined, and an interaction network, in which competitive and mutualistic interactions are modeled. From this second network, we can begin to disentangle the different types of competitive mutualistic interactions. If we combine the insights from these networks with in vitro (petri dish) results on certain interactions (say it's known that one bacteria strain produces a product that inhibits its competition from going after the same food), we can get even further in untangling the complex interactions in our gut.

It’s important to understand that the microbiome is so complex that no single technique will be able to map every interaction. Making networks from microbiome samples using statistics gives information on the real-life interactions in the gut, but it is difficult to determine the exact ways in which these microbes interact. Isolating certain microbes and mapping their interactions in a lab gives exact information on how two microorganisms interact, but these interactions may change in the dynamic environment of our guts.
That’s why having a complete picture of our guts is so difficult and has been a booming field of research in recent years. Combining purely statistical methods, like creating co-occurrence networks with biological knowledge, has strong promise. It’s invigorating to imagine what could be possible if we were able to completely map all the interactions in our guts at a given point in time. We could improve our health not by trying to eliminate certain bacteria but by influencing the neighboring microorganisms and regaining stability in our guts. This type of information could completely change the way we treat certain diseases, like obesity, IBS, among many others. Though this future seems very promising we aren’t there yet, and so we must just settle for having a piece of the puzzle, in the form of a co-occurrence or interaction network, and hope with time we will have enough pieces to build the puzzle.